Abstract
Background
The management of patients with refractory or relapsed Hodgkin lymphoma (HL), especially after autologous stem cell transplantation (ASCT), remains controversial. Bendamustine has demonstrated efficacy in several lymphoproliferative disorders but limited data are available regarding the schedule in patients with HL, in particular its dosage and the possible combinations for a synergistic effect. Brentuximab Vedotin is a CD30-directed antibody-drug conjugate, currently approved for the treatment of relapsed or refractory HL.
Aims
The objective of this retrospective observational trial was to evaluate efficacy and safety of salvage cytotoxic regimens in patients with refractory and/or relapsed HL. Three different regimens were evaluated.
Methods
From May 2011 to December 2016, 32 consecutive patients (19 M/13 F) with a median age of 31.7 years (range, 16-73) underwent to a salvage regimen after failure of ASCT. Patients were by chance assigned to one of these three arms: standard dose bendamustine (90 mg/sqm) days 1 and 2 plus standard DHAP schedule (every 4 weeks) x 3 cycles (Arm A, n= 10 cases), brentuximab single agent 1.8 mg/kg (every 3 weeks) x 4-8 cycles (Arm B, n= 11 cases), high dose bendamustine (120 mg/sqm) days 1 and 2 plus brentuximab 1.8 mg/kg (day 3) x 4-6 cycles (Arm C, n= 11 cases). Each cycle in arm C was repeated every 28 days and growth factor support was systematically administered, in association with antimicrobial prophylaxis. The treatment efficacy in each arm was evaluated according to Revised Response Criteria for Malignant Lymphoma by Cheson et al. Any adverse event occurred was recorded and classified for type and grade using NCI-CTCAE criteria (v 4.0).
Results
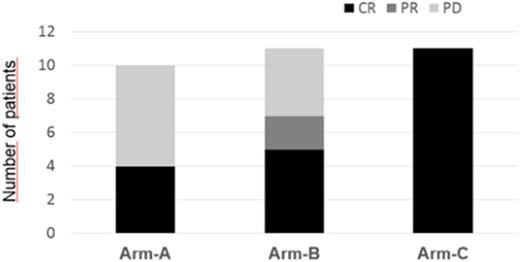
In arm A, the overall response rate (ORR) was 40% (4/10 patients), with 4 (40%) complete remission (CR) and 6 (60%) progressive disease (PD). Hematological toxicity was grade 3 thrombocytopenia in 4 patients (40%) and bone marrow aplasia in 1 patient (10%); extra-hematological toxicity was gastrointestinal toxicity of grade 2 in 6 patients (60%) and grade 1 in 3 patients (30%), In arm B, ORR was 63.6% (7/11 patients), with 5 (45%) CR, 2 (18%) partial response (PR) and 4 (36%) PD. Hematological toxicity was grade 2 neutropenia in 4 patients (36%), extra-hematological toxicity was grade 3 neuropathy in 2 patients (18%), In arm C, ORR was 100% (11/11 patients), with 11 CR followed by SCT (second autologous transplant, 6 cases; and haploidentical transplant, 5 cases) with persistence of complete remission in all patients at a median follow-up of 33.4 months (range, 12-60). Hematological toxicity was grade 3 thrombocytopenia in 4 patients (36.3%); extra-hematological toxicities were increase of transaminase (grade 2) in 3 patients (27%) and cytomegalovirus (CMV) reactivation in 2 patients (18%), treated successfully with valganciclovir. Three patients had fever during infusion at first cycle, together with a skin rash, managed with corticosteroid injections, and a successful antihistamine plus corticosteroid prophylaxis in the next cycles of treatment.
Conclusions
High-dose bendamustine plus brentuximab has shown relevant efficacy and a relatively good safety profile in a setting of heavily pretreated patients with HL. Adequate monitoring of CMV reactivation is recommended. This combination could be considered as a bridge to second autologous or allogenic SCT. However, these results should be validated by controlled and prospective studies involving larger number of patients.
Pane: Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal